Revolutionize Medical Device Industry with Sustainable Innovation

- Surendiran R
- June 20, 2023
The healthcare industry, alternatively referred to as the medical industry or the health economy, encompasses a diverse range of economic sectors dedicated to providing products and services aimed at addressing the various needs of patients across curative, preventive, rehabilitative, and palliative care domains. To address the demands of individuals and society, interdisciplinary teams of skilled professionals and para professionals work as part of the modern healthcare industry, encompassing three essential branches: services, products, and financing.
The healthcare sector is among the largest and fastest growing in the world. It accounts for more than 10% of the GDP in many developed countries, a significant portion of the economy.
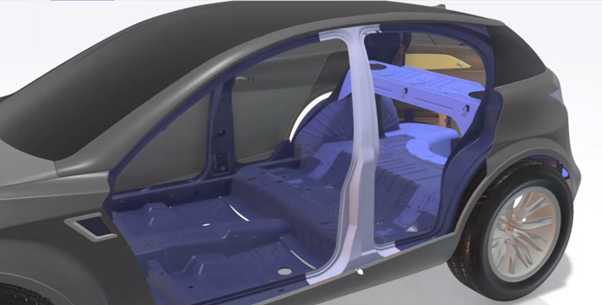
Remarkable technological advancements have been made in the healthcare sector to extend and improve the quality of life for many people. Things that were supposed to be inconceivable a few years ago are now coming to pass. The product development of medical devices has a bright future. However, there are a lot of challenges. Here are some challenges that will be faced when bringing new devices to market.
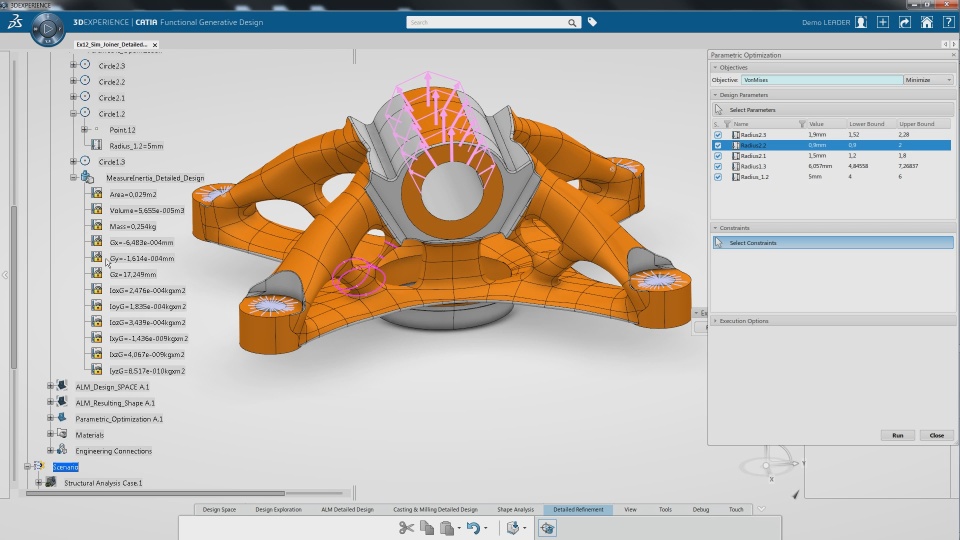
Accelerate the Product Development Speed with Integrated Interface for Modeling & Simulation
Modeling and simulation create more design options in a low-risk and low-cost environment faster. Each product lifecycle stage is optimized for speed and efficiency through democratization beyond the specialists to help companies:
- Understand the physics affecting device performance to comply with Statutory requirements
- Expedite testing and approval processes with alternatives to costly animal and human testing
- Streamline manufacturing processes for faster & smarter decision making

Product Complexity and Change Management while Reducing the Risk of Non-Compliance
The process of handling quality issues such as Corrective and Preventive Actions and product complaints is the single most significant source of regulatory risk for medical device manufacturers today worldwide.
Effective and efficient management of quality issues by improving traceability and compliance to industry standards and QMS while eliminating non-value-added activities to reduce waste and deliver unmatched quality, safety and potency reduces regulatory risks and enhances compliance.
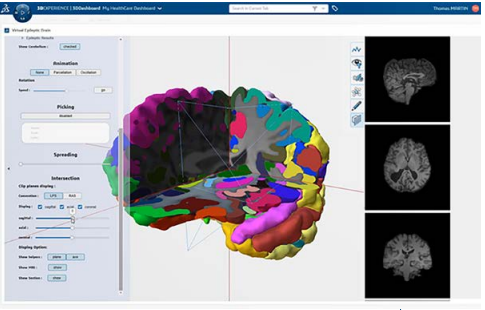
Deliver Patient-Centric Experiences
Medical device manufacturers are investigating ideas to deliver superior personalized, patient-centric experiences that improve patient health. This shift in innovation is focused on both the therapies and medical technologies they create and the processes that support their ecosystem.
Delivering life-like, multi-scale and multi-physic models, enabling an end-to-end virtual environment for accelerated collaborative innovation, is one of the predominant challenges that medical device manufacturers face in current scenarios.
Knowledge Capitalization
Medical device manufacturing companies operate in numerous isolated divisions. To manage this, many organizations have structured, complex, matrix-based organization structures attempting to enhance cross-division communication and data exchange to streamline internal processes. However, the scope is beyond the actual requirements and norms under compliance and regulations.
The digitalization of businesses that leverage continuity across the entire innovation team will address this challenge. This will transform how they innovate and operate, driving significantly enhanced margins with patient-centric experiences and increased productivity and profits.
Revitalize the Value Chain
Healthcare companies look to enhance their competition by accelerating innovation, maximizing ROI and creating new, connected experiences for their patients. Business leaders see significant growth in collaborative invention, and new models will emerge throughout the manufacturing value chain and traditional supply chains.
Transform Development & Manufacturing Operations
Decision makers or stakeholders in the healthcare industry must continually evaluate how to improve manufacturing processes to drive efficiency, quality, and performance. Leveraging digital design and production processes presents an opportunity to accelerate innovation and new product introduction.
Setting up digital manufacturing, planning and execution solutions, delivering agile manufacturing and planning operations, and offering real-time visibility and control over the business processes performed by plants and suppliers are some of the critical challenges that need to be addressed to run the development process efficiently.
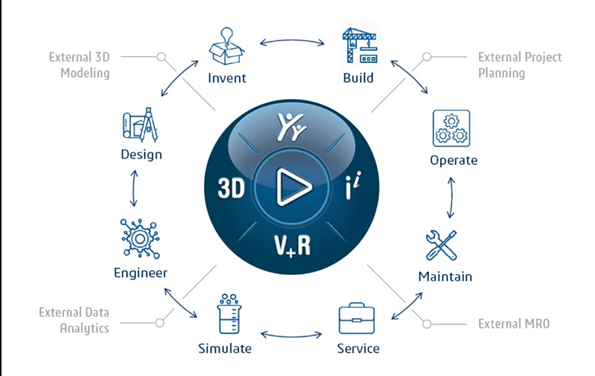
Dassault Systèmes’ 3DEXPERIENCE Platform is a one stop solution which combines engineering, quality and regulatory compliance business processes. Companies can accelerate the product development process, enhance innovation, and deliver products in compliance with regulatory norms and patient-centric approach by implementing the digital experience platform.
To get more information on how the 3DEXPERIENCE Platform drives the innovation in the medical industry, please reach out to us at marketing@edstechnologies.com

- Surendiran R
- June 20, 2023
Revolutionize Medical Device Industry with Sustainable Innovation
The healthcare industry, alternatively referred to as the medical industry or the health economy, encompasses a diverse range of economic sectors dedicated to providing products and services aimed at addressing the various needs of patients across curative, preventive, rehabilitative, and palliative care domains. To address the demands of individuals and society, interdisciplinary teams of skilled professionals and para professionals work as part of the modern healthcare industry, encompassing three essential branches: services, products, and financing.
The healthcare sector is among the largest and fastest growing in the world. It accounts for more than 10% of the GDP in many developed countries, a significant portion of the economy.
Remarkable technological advancements have been made in the healthcare sector to extend and improve the quality of life for many people. Things that were supposed to be inconceivable a few years ago are now coming to pass. The product development of medical devices has a bright future. However, there are a lot of challenges. Here are some challenges that will be faced when bringing new devices to market.
Accelerate the Product Development Speed with Integrated Interface for Modeling & Simulation
Modeling and simulation create more design options in a low-risk and low-cost environment faster. Each product lifecycle stage is optimized for speed and efficiency through democratization beyond the specialists to help companies:
- Understand the physics affecting device performance to comply with Statutory requirements
- Expedite testing and approval processes with alternatives to costly animal and human testing
- Streamline manufacturing processes for faster & smarter decision making

Product Complexity and Change Management while Reducing the Risk of Non-Compliance
The process of handling quality issues such as Corrective and Preventive Actions and product complaints is the single most significant source of regulatory risk for medical device manufacturers today worldwide.
Effective and efficient management of quality issues by improving traceability and compliance to industry standards and QMS while eliminating non-value-added activities to reduce waste and deliver unmatched quality, safety and potency reduces regulatory risks and enhances compliance.

Deliver Patient-Centric Experiences
Medical device manufacturers are investigating ideas to deliver superior personalized, patient-centric experiences that improve patient health. This shift in innovation is focused on both the therapies and medical technologies they create and the processes that support their ecosystem.
Delivering life-like, multi-scale and multi-physic models, enabling an end-to-end virtual environment for accelerated collaborative innovation, is one of the predominant challenges that medical device manufacturers face in current scenarios.
Knowledge Capitalization
Medical device manufacturing companies operate in numerous isolated divisions. To manage this, many organizations have structured, complex, matrix-based organization structures attempting to enhance cross-division communication and data exchange to streamline internal processes. However, the scope is beyond the actual requirements and norms under compliance and regulations.
The digitalization of businesses that leverage continuity across the entire innovation team will address this challenge. This will transform how they innovate and operate, driving significantly enhanced margins with patient-centric experiences and increased productivity and profits.
Revitalize the Value Chain
Healthcare companies look to enhance their competition by accelerating innovation, maximizing ROI and creating new, connected experiences for their patients. Business leaders see significant growth in collaborative invention, and new models will emerge throughout the manufacturing value chain and traditional supply chains.
Transform Development & Manufacturing Operations
Decision makers or stakeholders in the healthcare industry must continually evaluate how to improve manufacturing processes to drive efficiency, quality, and performance. Leveraging digital design and production processes presents an opportunity to accelerate innovation and new product introduction.
Setting up digital manufacturing, planning and execution solutions, delivering agile manufacturing and planning operations, and offering real-time visibility and control over the business processes performed by plants and suppliers are some of the critical challenges that need to be addressed to run the development process efficiently.
Dassault Systèmes’ 3DEXPERIENCE Platform is a one stop solution which combines engineering, quality and regulatory compliance business processes. Companies can accelerate the product development process, enhance innovation, and deliver products in compliance with regulatory norms and patient-centric approach by implementing the digital experience platform.
To get more information on how the 3DEXPERIENCE Platform drives the innovation in the medical industry, please reach out to us at marketing@edstechnologies.com