Leveraging SIMULIA for Medical Device Innovation
- Varun Sharma
- December 26, 2024
The medical device industry requires innovation, precision, and compliance to deliver safe, effective, and reliable products. SIMULIA, as part of Dassault Systèmes’ 3DEXPERIENCE platform, enables engineers to simulate real-world behaviours and optimize designs across various applications. This ensures reduced costs, accelerated development, and compliance with stringent regulatory requirements.
Why SIMULIA for Medical Devices?
SIMULIA provides a comprehensive platform for conducting realistic simulations across multiple physics domains, including structural, thermal, and fluid dynamics. This capability is crucial for medical devices, which must operate reliably under complex physiological conditions. Below are some ways SIMULIA supports the development of cutting-edge medical devices:
- Improved Design Efficiency: By simulating device performance virtually, developers can reduce the need for physical prototypes, saving time and costs.
- Enhanced Safety and Reliability: SIMULIA helps predict how devices behave under various loading conditions, ensuring they meet safety and regulatory requirements.
- Multiphysics Integration: Medical devices often involve coupled physics, such as heat transfer in surgical tools or fluid-structure interaction in cardiovascular devices. SIMULIA’s tools enable seamless integration of these effects.
- Optimization and Customization: With tools like Tosca and Isight, SIMULIA enables the optimization of device design for performance, weight, and material usage. Please refer below detailed image about SIMULIA.
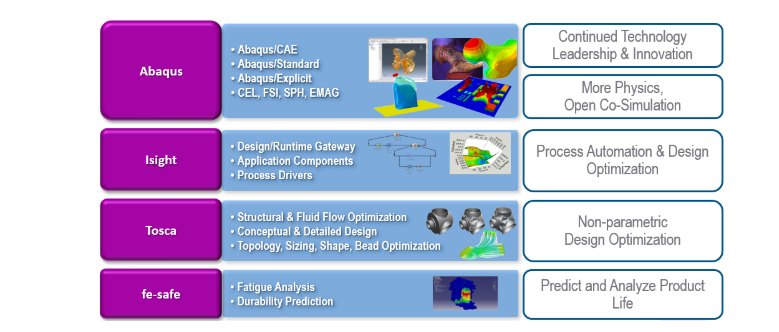
Key Applications of SIMULIA in Medical Device Development
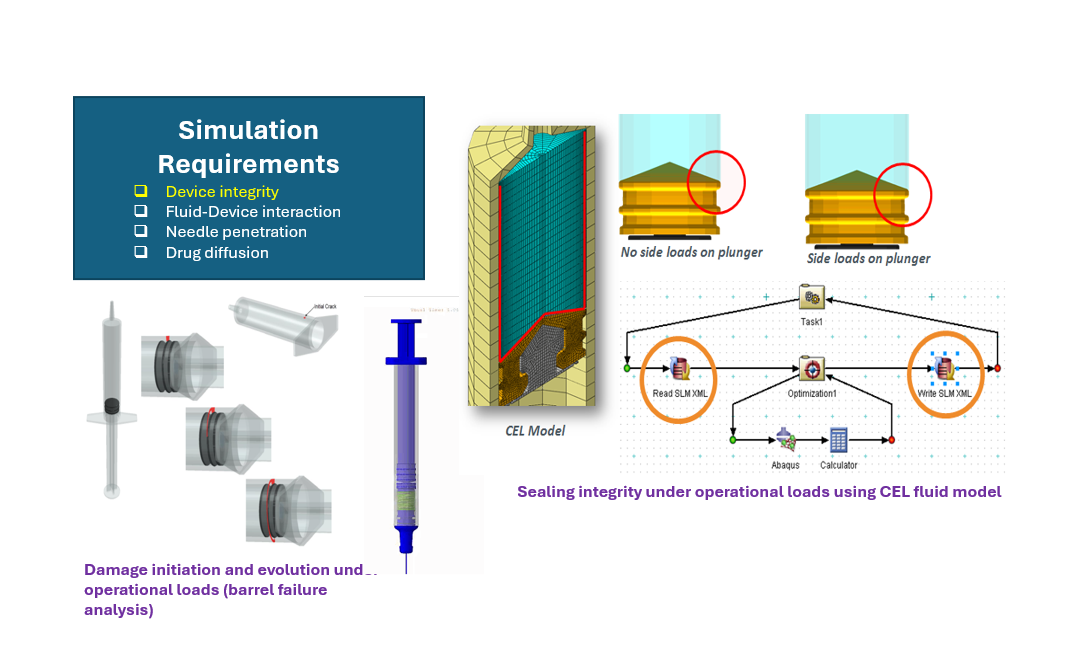
Needle Penetration in Skin
- Description: SIMULIA provides the capability to simulate needle penetration through skin layers, including tissue damage modelling, fluid interactions, and drug diffusion.
- Simulation Requirements:
- Device integrity and durability.
- Fluid-device interaction (FSI) for accurate diffusion modelling.
- Realistic penetration dynamics and soft tissue deformation.
- Drug diffusion analysis for controlled delivery.
- Example Use Case:
- Simulating a hypodermic needle to understand tissue damage and optimize injection depth while ensuring minimal patient discomfort.

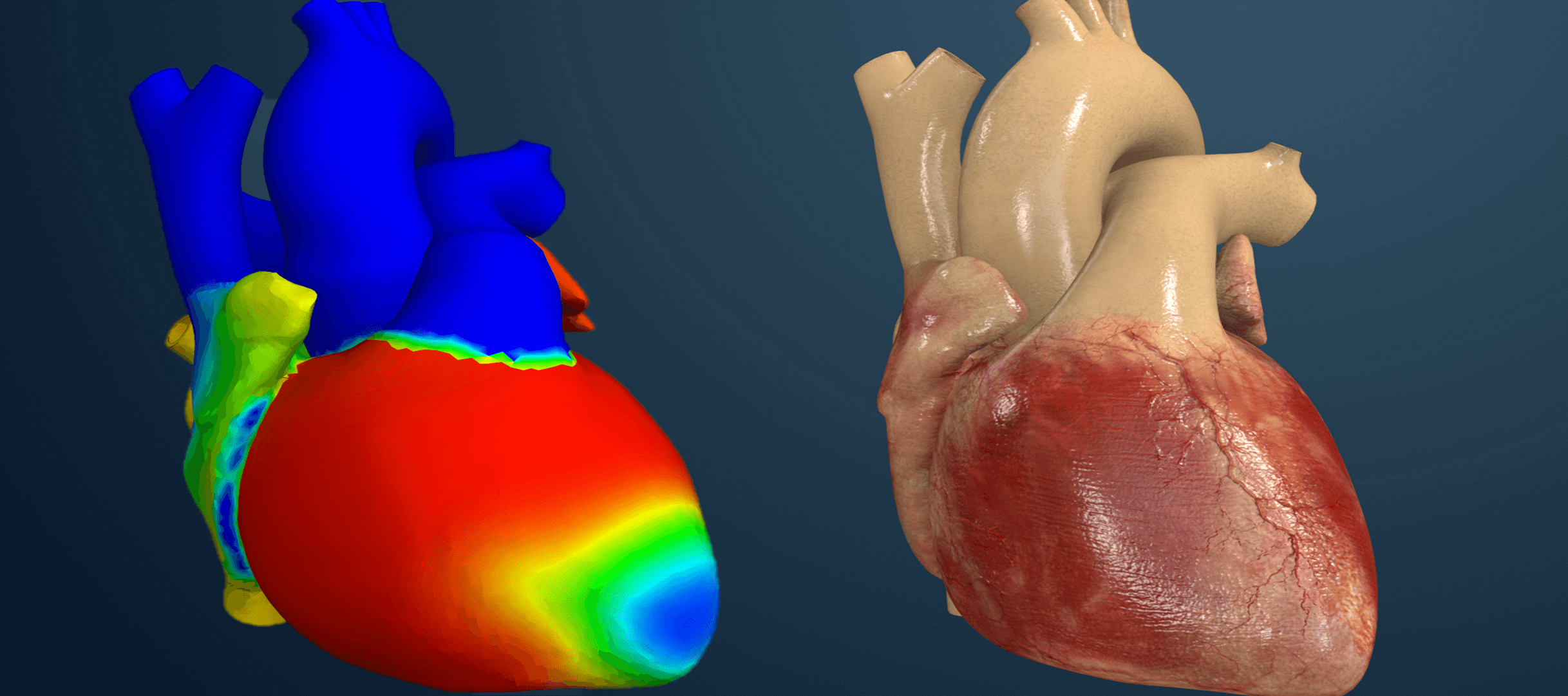
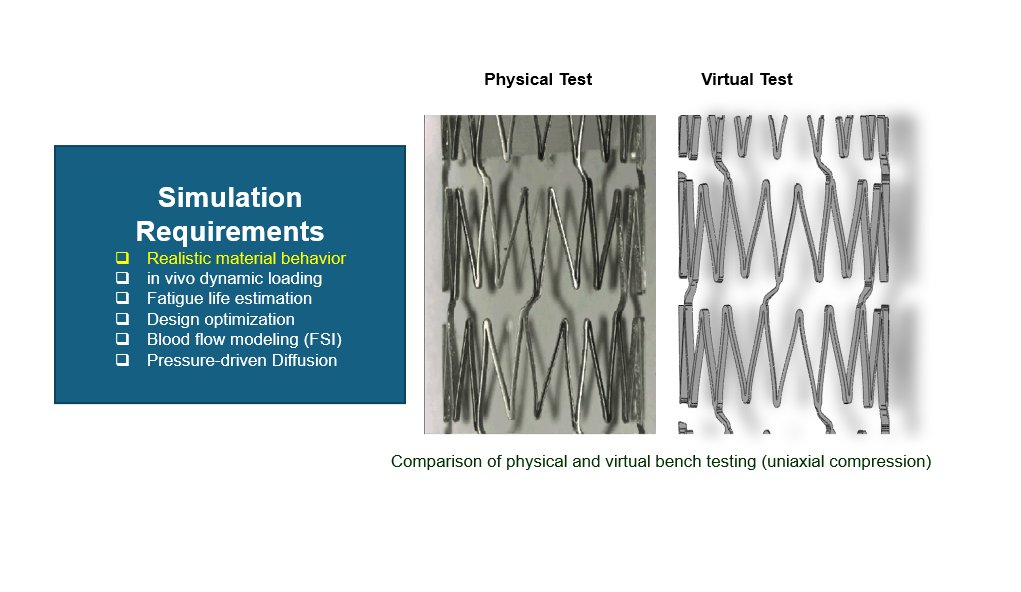
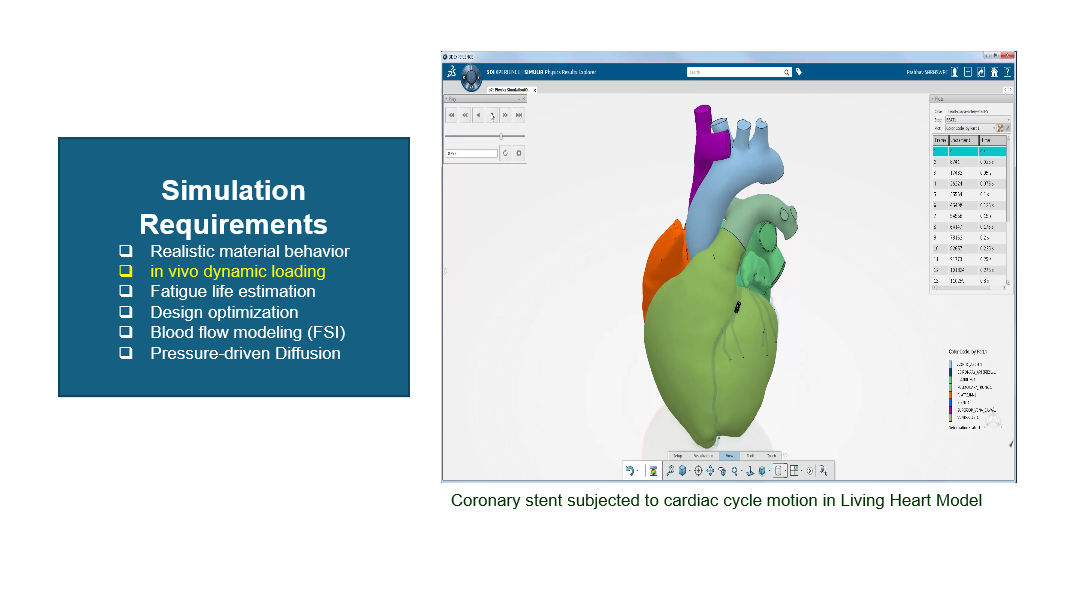
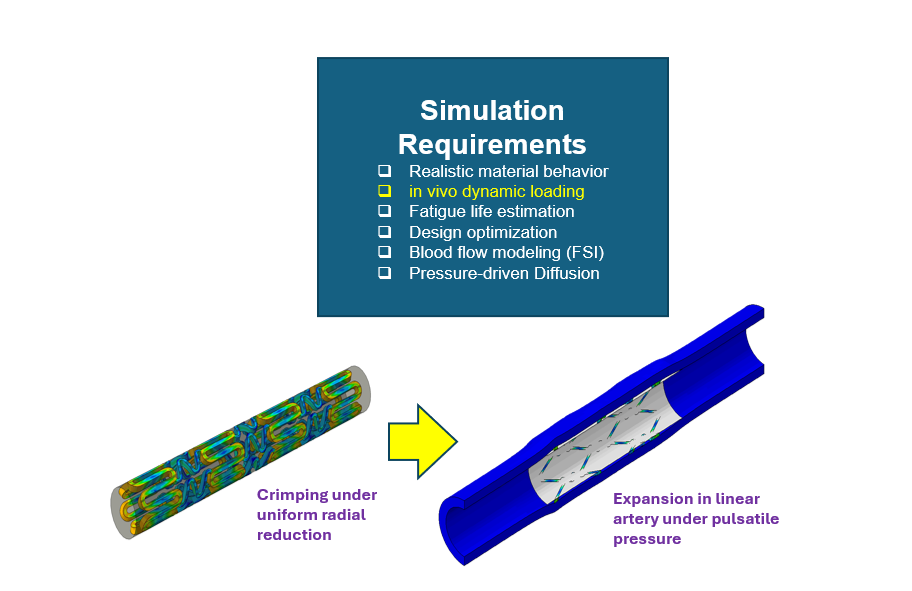
Coronary Stent Design
- Description: Coronary stents are critical devices for treating narrowed arteries. SIMULIA enables stent crimping, balloon expansion, and arterial interaction simulations to validate their design.
- Simulation Requirements:
-
- Realistic material behaviour for stents under in-vivo dynamic loading.
- Fatigue life estimation to ensure long-term reliability under cardiac cycles.
- Design optimization to reduce failure risks and enhance performance.
- Blood flow modelling using Fluid-Structure Interaction (FSI).
- Diffusion modelling for pressure-driven drug release.
- Example Use Case:
-
- Crimping the stent under uniform radial reduction and expanding it inside a pulsatile pressure artery to evaluate its structural integrity and fatigue life.
Peripheral Stent Simulation
- Description: Peripheral stents face complex deformations due to arterial movements. SIMULIA simulates these arterial deformations to optimize stent performance.
- Simulation Requirements:
- Realistic arterial behaviour under motion and pressure changes.
- Fatigue and durability assessments.
- Expansion dynamics in non-linear arteries derived from patient data.
- Example Use Case:
- Evaluating peripheral stents’ interaction with arteries under varying deformation profiles to minimize failure.
Drug Delivery Systems
- Description: Drug delivery devices, such as combination products, require simulations for diffusion, fluid-device interaction, and controlled release mechanisms.
- Simulation Requirements:
- Pressure-driven drug diffusion analysis.
- Coupled fluid-structure dynamics for delivery accuracy.
- Multiphysics simulations for combination products involving mechanical and chemical interactions.
- Example Use Case:
- Optimizing drug delivery systems to ensure precise dosage and controlled diffusion based on physiological conditions.
Balloon Expansion in Patient-Derived Arteries
- Description: Balloon catheters require accurate simulations to evaluate expansion behaviour in patient-derived artery geometries.
- Simulation Requirements:
- Arterial material modelling for realistic deformation.
- Expansion analysis using coupled mechanical and pressure-driven simulations.
- Predicting device failure points under non-linear artery shapes.
- Example Use Case:
- Simulating guide-wire insertion and balloon expansion to evaluate catheter behaviour under dynamic conditions.
Human Factors Design
- Description: SIMULIA supports the design of medical devices that account for ergonomics and end-user experiences. This ensures devices are intuitive and user-friendly while meeting performance criteria.
- Simulation Requirements:
- Industrial design for optimal ergonomics.
- Analysis of human-device interaction using virtual human models.
- Example Use Case:
- Designing surgical tools with improved usability to reduce fatigue for surgeons and healthcare workers.
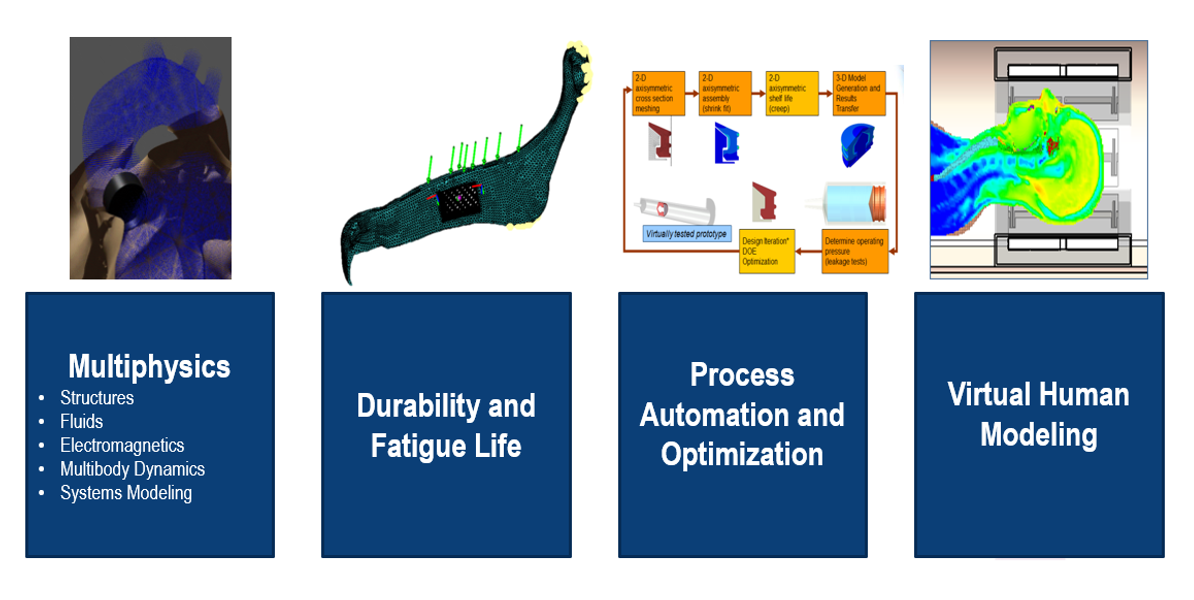
Combination Products and Multiphysics Simulation
The fast-growing combination product market integrates therapeutics, drug delivery systems, and mechanical devices. SIMULIA’s Multiphysics and multiscale simulations are uniquely suited to address:
- Accurate prediction of device-drug interactions.
- Virtual human modelling for patient-specific simulations.
- Design validation for safe and effective products.
Advanced Visualization and Colour Contour Analysis
SIMULIA’s visualization tools provide clear insights into complex simulations through colour contour analysis. These outputs allow engineers to:
- Identify areas of high stress, strain, or temperature.
- Optimize designs for safety and reliability.
- Validate performance using realistic models.
Benefits of SIMULIA for Medical Devices
- Accelerated Development: Reduce reliance on physical prototypes through virtual testing.
- Improved Safety: Simulate real-world conditions to ensure devices perform safely.
- Regulatory Compliance: Meet strict industry standards with validated simulation results.
- Cost Reduction: Optimize materials, design, and manufacturing processes.
- Patient-Specific Solutions: Use patient-derived data for personalized device design.
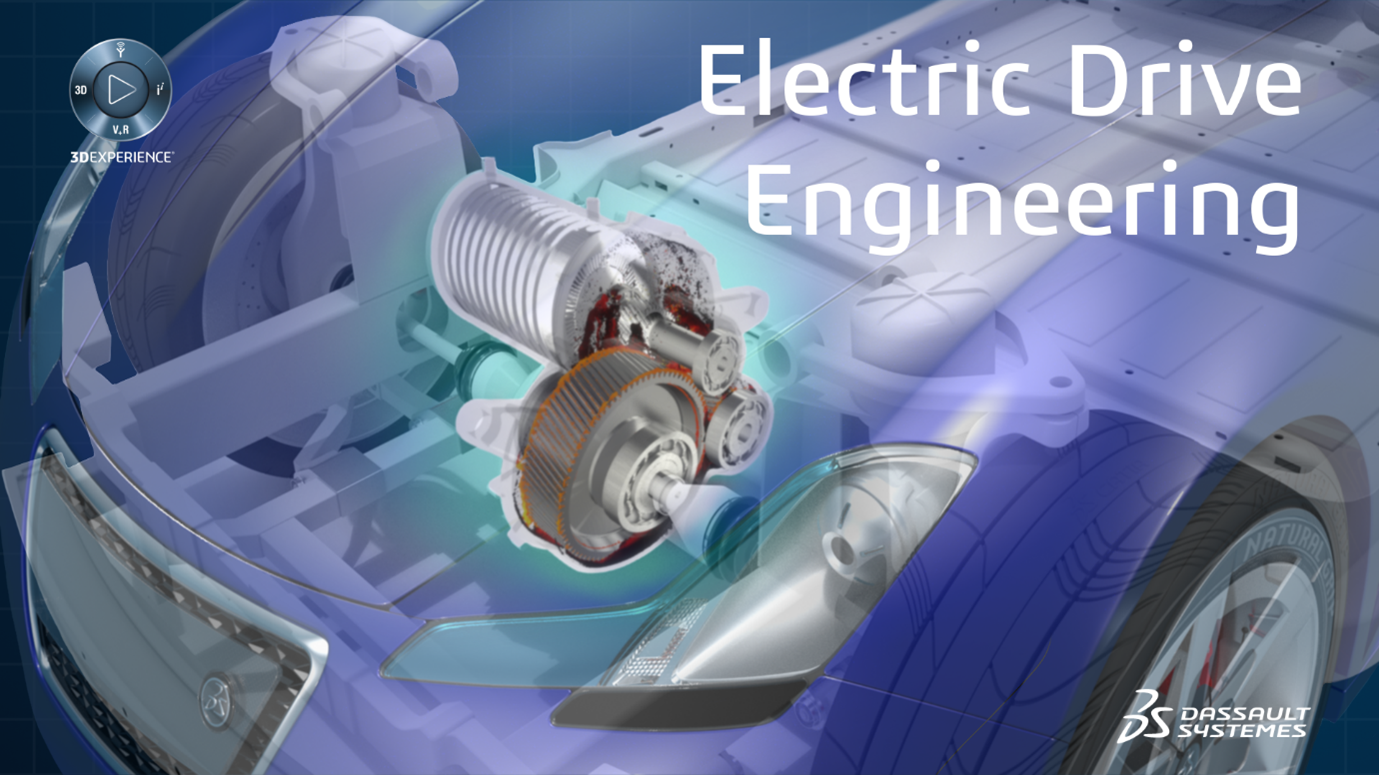
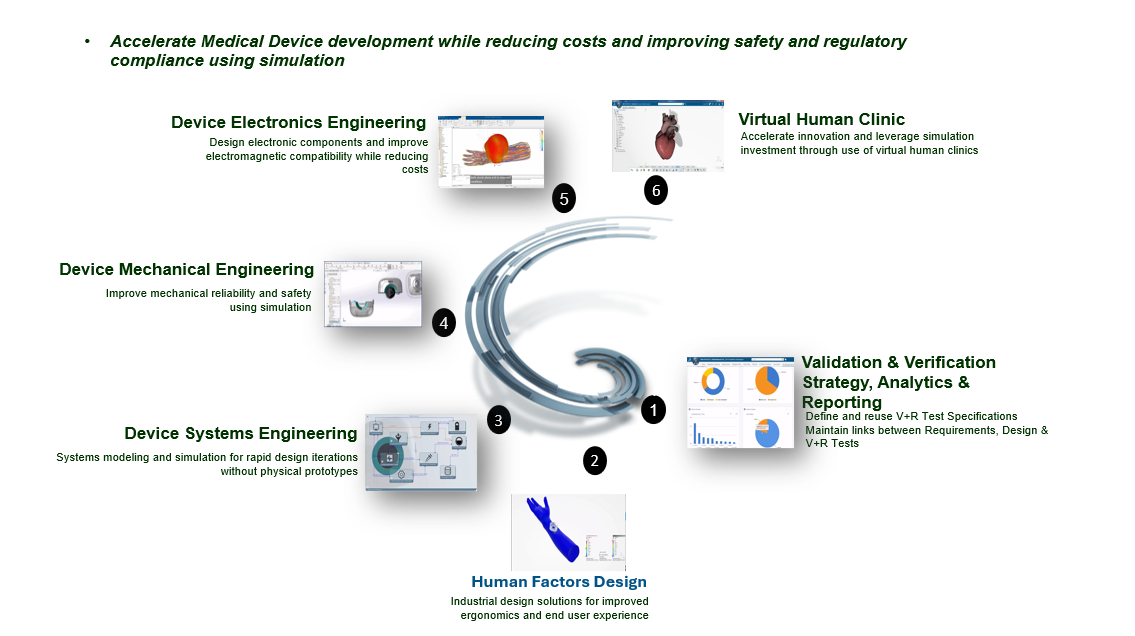
Key Features Shown in Image
- Validation & Verification Strategy, Analytics & Reporting – Ensuring design compliance and reliability.
- Human Factors Design – Industrial solutions for improved ergonomics.
- Device Systems Engineering – Rapid modelling and simulations.
- Device Mechanical Engineering – Enhancing mechanical safety.
- Device Electronics Engineering – Optimizing electronic components and electromagnetic compatibility.
- Virtual Human Clinic – Simulating human body interactions with devices for enhanced innovation.
Conclusion
SIMULIA revolutionizes the medical device industry by enabling advanced simulation, realistic modelling, and optimization across various applications. Whether it’s needle penetration, coronary stents, or drug delivery systems, SIMULIA provides the tools to develop safer, cost-effective, and high-performing devices.
With Multiphysics capabilities and integration into the 3DEXPERIENCE platform, SIMULIA empowers organizations to achieve innovation faster and deliver devices that transform patient care.

- Varun Sharma
- December 26, 2024
Leveraging SIMULIA for Medical Device Innovation
The medical device industry requires innovation, precision, and compliance to deliver safe, effective, and reliable products. SIMULIA, as part of Dassault Systèmes’ 3DEXPERIENCE platform, enables engineers to simulate real-world behaviours and optimize designs across various applications. This ensures reduced costs, accelerated development, and compliance with stringent regulatory requirements.
Why SIMULIA for Medical Devices?
SIMULIA provides a comprehensive platform for conducting realistic simulations across multiple physics domains, including structural, thermal, and fluid dynamics. This capability is crucial for medical devices, which must operate reliably under complex physiological conditions. Below are some ways SIMULIA supports the development of cutting-edge medical devices:
- Improved Design Efficiency: By simulating device performance virtually, developers can reduce the need for physical prototypes, saving time and costs.
- Enhanced Safety and Reliability: SIMULIA helps predict how devices behave under various loading conditions, ensuring they meet safety and regulatory requirements.
- Multiphysics Integration: Medical devices often involve coupled physics, such as heat transfer in surgical tools or fluid-structure interaction in cardiovascular devices. SIMULIA’s tools enable seamless integration of these effects.
- Optimization and Customization: With tools like Tosca and Isight, SIMULIA enables the optimization of device design for performance, weight, and material usage. Please refer below detailed image about SIMULIA.

Key Applications of SIMULIA in Medical Device Development
Needle Penetration in Skin
- Description: SIMULIA provides the capability to simulate needle penetration through skin layers, including tissue damage modelling, fluid interactions, and drug diffusion.
- Simulation Requirements:
- Device integrity and durability.
- Fluid-device interaction (FSI) for accurate diffusion modelling.
- Realistic penetration dynamics and soft tissue deformation.
- Drug diffusion analysis for controlled delivery.
- Example Use Case:
- Simulating a hypodermic needle to understand tissue damage and optimize injection depth while ensuring minimal patient discomfort.

Coronary Stent Design
- Description: Coronary stents are critical devices for treating narrowed arteries. SIMULIA enables stent crimping, balloon expansion, and arterial interaction simulations to validate their design.
- Simulation Requirements:
-
- Realistic material behaviour for stents under in-vivo dynamic loading.
- Fatigue life estimation to ensure long-term reliability under cardiac cycles.
- Design optimization to reduce failure risks and enhance performance.
- Blood flow modelling using Fluid-Structure Interaction (FSI).
- Diffusion modelling for pressure-driven drug release.
- Example Use Case:
-
- Crimping the stent under uniform radial reduction and expanding it inside a pulsatile pressure artery to evaluate its structural integrity and fatigue life.

Peripheral Stent Simulation
- Description: Peripheral stents face complex deformations due to arterial movements. SIMULIA simulates these arterial deformations to optimize stent performance.
- Simulation Requirements:
- Realistic arterial behaviour under motion and pressure changes.
- Fatigue and durability assessments.
- Expansion dynamics in non-linear arteries derived from patient data.
- Example Use Case:
- Evaluating peripheral stents’ interaction with arteries under varying deformation profiles to minimize failure.

Drug Delivery Systems
- Description: Drug delivery devices, such as combination products, require simulations for diffusion, fluid-device interaction, and controlled release mechanisms.
- Simulation Requirements:
- Pressure-driven drug diffusion analysis.
- Coupled fluid-structure dynamics for delivery accuracy.
- Multiphysics simulations for combination products involving mechanical and chemical interactions.
- Example Use Case:
- Optimizing drug delivery systems to ensure precise dosage and controlled diffusion based on physiological conditions.

Balloon Expansion in Patient-Derived Arteries
- Description: Balloon catheters require accurate simulations to evaluate expansion behaviour in patient-derived artery geometries.
- Simulation Requirements:
- Arterial material modelling for realistic deformation.
- Expansion analysis using coupled mechanical and pressure-driven simulations.
- Predicting device failure points under non-linear artery shapes.
- Example Use Case:
- Simulating guide-wire insertion and balloon expansion to evaluate catheter behaviour under dynamic conditions.

Human Factors Design
- Description: SIMULIA supports the design of medical devices that account for ergonomics and end-user experiences. This ensures devices are intuitive and user-friendly while meeting performance criteria.
- Simulation Requirements:
- Industrial design for optimal ergonomics.
- Analysis of human-device interaction using virtual human models.
- Example Use Case:
- Designing surgical tools with improved usability to reduce fatigue for surgeons and healthcare workers.
Combination Products and Multiphysics Simulation
The fast-growing combination product market integrates therapeutics, drug delivery systems, and mechanical devices. SIMULIA’s Multiphysics and multiscale simulations are uniquely suited to address:
- Accurate prediction of device-drug interactions.
- Virtual human modelling for patient-specific simulations.
- Design validation for safe and effective products.
Advanced Visualization and Colour Contour Analysis
SIMULIA’s visualization tools provide clear insights into complex simulations through colour contour analysis. These outputs allow engineers to:
- Identify areas of high stress, strain, or temperature.
- Optimize designs for safety and reliability.
- Validate performance using realistic models.

Benefits of SIMULIA for Medical Devices
- Accelerated Development: Reduce reliance on physical prototypes through virtual testing.
- Improved Safety: Simulate real-world conditions to ensure devices perform safely.
- Regulatory Compliance: Meet strict industry standards with validated simulation results.
- Cost Reduction: Optimize materials, design, and manufacturing processes.
- Patient-Specific Solutions: Use patient-derived data for personalized device design.
Key Features Shown in Image
- Validation & Verification Strategy, Analytics & Reporting – Ensuring design compliance and reliability.
- Human Factors Design – Industrial solutions for improved ergonomics.
- Device Systems Engineering – Rapid modelling and simulations.
- Device Mechanical Engineering – Enhancing mechanical safety.
- Device Electronics Engineering – Optimizing electronic components and electromagnetic compatibility.
- Virtual Human Clinic – Simulating human body interactions with devices for enhanced innovation.

Conclusion
SIMULIA revolutionizes the medical device industry by enabling advanced simulation, realistic modelling, and optimization across various applications. Whether it’s needle penetration, coronary stents, or drug delivery systems, SIMULIA provides the tools to develop safer, cost-effective, and high-performing devices.
With Multiphysics capabilities and integration into the 3DEXPERIENCE platform, SIMULIA empowers organizations to achieve innovation faster and deliver devices that transform patient care.