FEA Simulation of Medical Devices Using SIMULIA

- Saifali Davalanavar
- December 26, 2024
The development of medical devices has seen significant advancements over the years, driven by innovations in technology, materials, and regulatory requirements. A crucial component in this process is the use of Finite Element Analysis (FEA) simulation. This powerful computational tool allows engineers to analyze, optimize, and validate medical device designs virtually, improving their performance, safety, and compliance with regulations before physical prototypes are created.
SIMULIA, a suite of advanced simulation tools developed by Dassault Systèmes, provides a comprehensive platform for FEA simulation, particularly in the medical device sector. In this blog, we’ll explore how FEA simulation using SIMULIA revolutionizes the design and testing of medical devices, its benefits, challenges, and real-world applications ensuring that they meet the strict regulatory requirements of the healthcare industry.
What is FEA and How Does it Work?
Finite Element Analysis (FEA) is a numerical method used to solve complex physical problems in engineering. By breaking down a structure into small, discrete elements (often referred to as a mesh), FEA can simulate the behavior of materials and components under various conditions like stress, vibration, temperature, and fluid flow. It is widely used in industries such as aerospace, automotive, and biomedical industries, to model how a device or system will behave in the real world.
The FEA simulation process involves:
- Creating a geometric model of the device e.g., a stent, prosthetic limb, or surgical instrument.
- Assigning material properties such as super elasticity for stents.
- Meshing the model into smaller, discrete elements.
- Creating interactions for defining the contact behavior between two or more parts and interaction of a part with itself during deformation.
- Applying boundary conditions such as load, displacement, velocity and restraints.
- Solving the equations governing the behavior of the system.
- Post-processing the results which may include visualizing deformation, stress distribution, and thermal effects.
In the context of medical devices, FEA simulation helps in assessing aspects such as:
- Mechanical performance: How the device holds up under stress and strain.
- Thermal performance: How temperature changes affect the device’s functionality.
- Fluid dynamics: How blood flow, or medication delivery impacts device operation.
- Failure analysis: Predicting potential points of failure or fatigue.
Why FEA Simulation is Crucial for Medical Devices
- Improved Design Validation
Medical devices, whether they are surgical instruments, implants, or diagnostic tools, must meet rigorous standards for safety and performance. FEA simulation helps design engineers validate their concepts long before physical prototypes are built. By simulating a device’s mechanical and physical properties, FEA provides early insights into design flaws or weaknesses that may not be apparent in early stages.
For instance, an orthopedic implant must be designed to withstand the forces of daily movement within the human body. FEA can simulate how the implant will behave under various stress scenarios, ensuring that it will not fail under typical conditions.
- Reduced Prototyping Costs and Time
Creating physical prototypes of medical devices for testing can be time-consuming and costly, especially when design iterations are frequent. FEA simulations allow for multiple design variations to be tested virtually, reducing the need for expensive and time-consuming physical prototypes. By optimizing the design through simulation, manufacturers can significantly reduce costs and accelerate the time to market.
- Enhanced Compliance with Regulatory Standards
Medical devices are heavily regulated by authorities such as the FDA (Food and Drug Administration) and EMA (European Medicines Agency). These agencies require manufacturers to demonstrate that their products meet strict safety and performance criteria before they can be approved for use. FEA simulations help manufacturers ensure that their devices meet regulatory requirements by providing robust, evidence-based analysis to support safety claims.
For example, in the case of a pacemaker, FEA can be used to simulate its behavior in the human body under various conditions (e.g., electrical conductivity, heat dissipation) to ensure it meets the required performance benchmarks for safety and efficacy.
- Optimization of Material Selection
The choice of materials for a medical device is critical. It must be biocompatible, durable, and functional under a variety of conditions. FEA simulation enables engineers to explore how different materials perform under various forces, temperatures, and other physical factors. Using SIMULIA’s tools, designers can simulate the behavior of different materials in the early stages of product development, ensuring optimal material selection for both performance and patient safety.
- Life Cycle Prediction and Durability Testing
Medical devices, especially implants and prosthetics, must function reliably over an extended period. SIMULIA’s advanced simulation capabilities allow for accelerated life cycle testing, providing insights into the durability of devices over time. FEA can predict wear and tear, fatigue, and potential failure modes, helping manufacturers optimize designs for longevity and reliability.
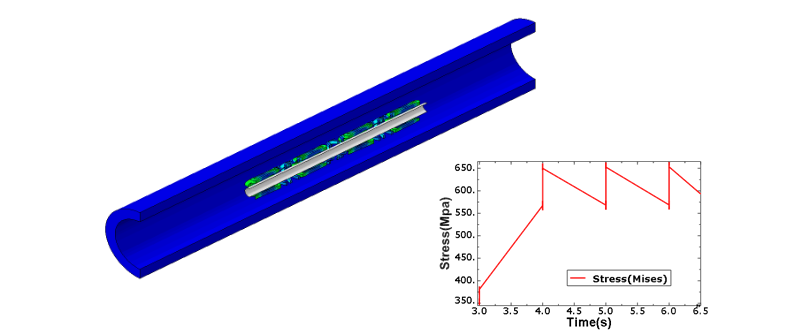
Fig. 1: Stent Cyclic Stresses/Strains – Fatigue Life due to blood pressure loading
- Predicting Complex Interactions in the Human Body
One of the most unique aspects of medical device design is the interaction between the device and the human body. SIMULIA allows engineers to simulate complex biomechanical interactions between medical devices and human tissues or organs. This is especially crucial for implants such as hip replacements, pacemakers, and dental devices. By modeling how a device will interact with biological materials, engineers can assess the potential for adverse reactions or complications, such as inflammation, tissue damage, or corrosion.
Real-World Applications of FEA in Medical Devices
- Orthopedic Implants and Prosthetics
In orthopedic applications, FEA is used to design and optimize implants such as hip replacements, knee joints, and spinal implants. By simulating the stress and strain on these implants under various conditions (e.g., walking, running, or lifting heavy weights), engineers can ensure the implants are durable and safe. For example, FEA can predict how a hip implant might interact with the surrounding bone and soft tissue, helping to design components that are less prone to wear and failure.
In prosthetics, FEA enables the creation of custom-made prosthetic limbs that provide a better fit for individual patients. The design can be adjusted based on specific factors such as weight distribution, movement range, and skin contact to maximize comfort and functionality.
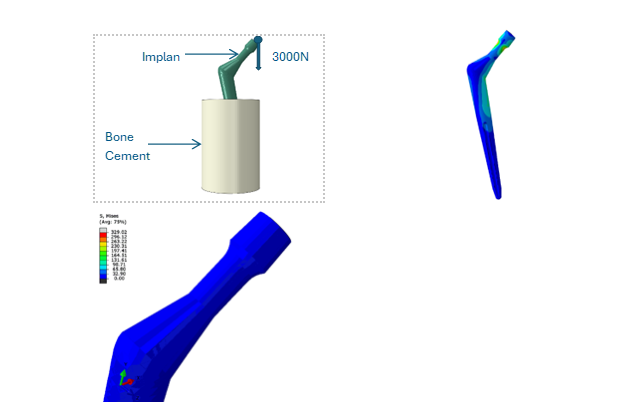
Fig. 2: Stresses in Hip implants undergone a standard test specified in ISO 7206/4
- Cardiovascular Devices (Stents, Valves, and Pacemakers)
For cardiovascular devices like stents, heart valves, and pacemakers, FEA is used to simulate the mechanical behavior of these devices in a human body. Stents, for example, need to expand in a controlled manner inside arteries without causing damage to the vessel walls. By using FEA to simulate the stent’s expansion, engineers can adjust the design to optimize performance and minimize risks.
Similarly, FEA is employed in the design of heart valves to simulate how the valve behaves under varying pressures and flow rates, ensuring that it functions effectively over a long period of time.
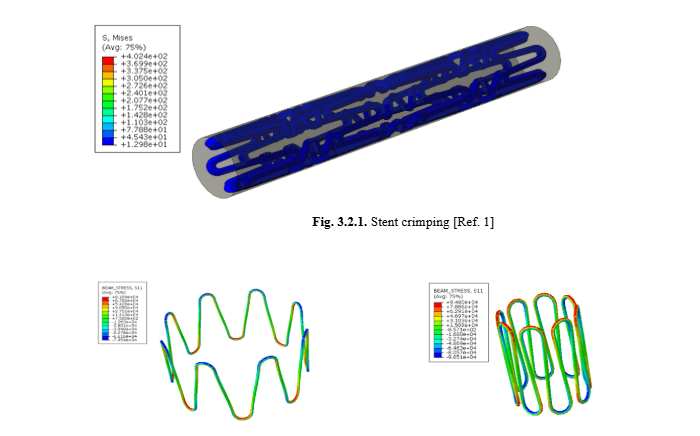
Fig. 3: Stent expansion and crimping
- Surgical Instruments
FEA is also instrumental in the design of surgical instruments, where factors such as precision, material strength, and ergonomics play a vital role. For example, surgical forceps or scalpels need to be designed for both strength and ease of use during complex procedures. FEA simulations help ensure that these tools will not break or deform during use and that they will be comfortable for the surgeon to handle.
- Dental Devices
In the dental industry, FEA simulations are used to design dental implants, crowns, and orthodontic devices. These simulations ensure that the devices can withstand the mechanical forces exerted during chewing, biting, and other activities. FEA also helps optimize the fit and comfort of dental devices by predicting how they will interact with the mouth’s soft tissues and bones.
Conclusion
The role of simulation, particularly FEA simulation using SIMULIA, has become an indispensable part of medical device design and testing. By enabling manufacturers to virtually test and optimize their products before they are built, SIMULIA ensures that medical devices are safe, reliable, and efficient. With its ability to simulate complex biological interactions, predict device performance, and streamline the design process, SIMULIA is driving the future of medical device innovation.
By leveraging these advanced tools, engineers can create medical devices that not only meet but exceed the expectations of patients, healthcare professionals, and regulatory bodies. The future of medical devices is undeniably linked to simulation, and SIMULIA is leading the way in transforming how these life-saving technologies are developed.

- Saifali Davalanavar
- December 26, 2024
FEA Simulation of Medical Devices Using SIMULIA
The development of medical devices has seen significant advancements over the years, driven by innovations in technology, materials, and regulatory requirements. A crucial component in this process is the use of Finite Element Analysis (FEA) simulation. This powerful computational tool allows engineers to analyze, optimize, and validate medical device designs virtually, improving their performance, safety, and compliance with regulations before physical prototypes are created.
SIMULIA, a suite of advanced simulation tools developed by Dassault Systèmes, provides a comprehensive platform for FEA simulation, particularly in the medical device sector. In this blog, we’ll explore how FEA simulation using SIMULIA revolutionizes the design and testing of medical devices, its benefits, challenges, and real-world applications ensuring that they meet the strict regulatory requirements of the healthcare industry.
What is FEA and How Does it Work?
Finite Element Analysis (FEA) is a numerical method used to solve complex physical problems in engineering. By breaking down a structure into small, discrete elements (often referred to as a mesh), FEA can simulate the behavior of materials and components under various conditions like stress, vibration, temperature, and fluid flow. It is widely used in industries such as aerospace, automotive, and biomedical industries, to model how a device or system will behave in the real world.
The FEA simulation process involves:
- Creating a geometric model of the device e.g., a stent, prosthetic limb, or surgical instrument.
- Assigning material properties such as super elasticity for stents.
- Meshing the model into smaller, discrete elements.
- Creating interactions for defining the contact behavior between two or more parts and interaction of a part with itself during deformation.
- Applying boundary conditions such as load, displacement, velocity and restraints.
- Solving the equations governing the behavior of the system.
- Post-processing the results which may include visualizing deformation, stress distribution, and thermal effects.
In the context of medical devices, FEA simulation helps in assessing aspects such as:
- Mechanical performance: How the device holds up under stress and strain.
- Thermal performance: How temperature changes affect the device’s functionality.
- Fluid dynamics: How blood flow, or medication delivery impacts device operation.
- Failure analysis: Predicting potential points of failure or fatigue.
Why FEA Simulation is Crucial for Medical Devices
- Improved Design Validation
Medical devices, whether they are surgical instruments, implants, or diagnostic tools, must meet rigorous standards for safety and performance. FEA simulation helps design engineers validate their concepts long before physical prototypes are built. By simulating a device’s mechanical and physical properties, FEA provides early insights into design flaws or weaknesses that may not be apparent in early stages.
For instance, an orthopedic implant must be designed to withstand the forces of daily movement within the human body. FEA can simulate how the implant will behave under various stress scenarios, ensuring that it will not fail under typical conditions.
- Reduced Prototyping Costs and Time
Creating physical prototypes of medical devices for testing can be time-consuming and costly, especially when design iterations are frequent. FEA simulations allow for multiple design variations to be tested virtually, reducing the need for expensive and time-consuming physical prototypes. By optimizing the design through simulation, manufacturers can significantly reduce costs and accelerate the time to market.
- Enhanced Compliance with Regulatory Standards
Medical devices are heavily regulated by authorities such as the FDA (Food and Drug Administration) and EMA (European Medicines Agency). These agencies require manufacturers to demonstrate that their products meet strict safety and performance criteria before they can be approved for use. FEA simulations help manufacturers ensure that their devices meet regulatory requirements by providing robust, evidence-based analysis to support safety claims.
For example, in the case of a pacemaker, FEA can be used to simulate its behavior in the human body under various conditions (e.g., electrical conductivity, heat dissipation) to ensure it meets the required performance benchmarks for safety and efficacy.
- Optimization of Material Selection
The choice of materials for a medical device is critical. It must be biocompatible, durable, and functional under a variety of conditions. FEA simulation enables engineers to explore how different materials perform under various forces, temperatures, and other physical factors. Using SIMULIA’s tools, designers can simulate the behavior of different materials in the early stages of product development, ensuring optimal material selection for both performance and patient safety.
- Life Cycle Prediction and Durability Testing
Medical devices, especially implants and prosthetics, must function reliably over an extended period. SIMULIA’s advanced simulation capabilities allow for accelerated life cycle testing, providing insights into the durability of devices over time. FEA can predict wear and tear, fatigue, and potential failure modes, helping manufacturers optimize designs for longevity and reliability.

Fig. 1: Stent Cyclic Stresses/Strains – Fatigue Life due to blood pressure loading
- Predicting Complex Interactions in the Human Body
One of the most unique aspects of medical device design is the interaction between the device and the human body. SIMULIA allows engineers to simulate complex biomechanical interactions between medical devices and human tissues or organs. This is especially crucial for implants such as hip replacements, pacemakers, and dental devices. By modeling how a device will interact with biological materials, engineers can assess the potential for adverse reactions or complications, such as inflammation, tissue damage, or corrosion.
Real-World Applications of FEA in Medical Devices
- Orthopedic Implants and Prosthetics
In orthopedic applications, FEA is used to design and optimize implants such as hip replacements, knee joints, and spinal implants. By simulating the stress and strain on these implants under various conditions (e.g., walking, running, or lifting heavy weights), engineers can ensure the implants are durable and safe. For example, FEA can predict how a hip implant might interact with the surrounding bone and soft tissue, helping to design components that are less prone to wear and failure.
In prosthetics, FEA enables the creation of custom-made prosthetic limbs that provide a better fit for individual patients. The design can be adjusted based on specific factors such as weight distribution, movement range, and skin contact to maximize comfort and functionality.

Fig. 2: Stresses in Hip implants undergone a standard test specified in ISO 7206/4
- Cardiovascular Devices (Stents, Valves, and Pacemakers)
For cardiovascular devices like stents, heart valves, and pacemakers, FEA is used to simulate the mechanical behavior of these devices in a human body. Stents, for example, need to expand in a controlled manner inside arteries without causing damage to the vessel walls. By using FEA to simulate the stent’s expansion, engineers can adjust the design to optimize performance and minimize risks.
Similarly, FEA is employed in the design of heart valves to simulate how the valve behaves under varying pressures and flow rates, ensuring that it functions effectively over a long period of time.

Fig. 3: Stent expansion and crimping
- Surgical Instruments
FEA is also instrumental in the design of surgical instruments, where factors such as precision, material strength, and ergonomics play a vital role. For example, surgical forceps or scalpels need to be designed for both strength and ease of use during complex procedures. FEA simulations help ensure that these tools will not break or deform during use and that they will be comfortable for the surgeon to handle.
- Dental Devices
In the dental industry, FEA simulations are used to design dental implants, crowns, and orthodontic devices. These simulations ensure that the devices can withstand the mechanical forces exerted during chewing, biting, and other activities. FEA also helps optimize the fit and comfort of dental devices by predicting how they will interact with the mouth’s soft tissues and bones.
Conclusion
The role of simulation, particularly FEA simulation using SIMULIA, has become an indispensable part of medical device design and testing. By enabling manufacturers to virtually test and optimize their products before they are built, SIMULIA ensures that medical devices are safe, reliable, and efficient. With its ability to simulate complex biological interactions, predict device performance, and streamline the design process, SIMULIA is driving the future of medical device innovation.
By leveraging these advanced tools, engineers can create medical devices that not only meet but exceed the expectations of patients, healthcare professionals, and regulatory bodies. The future of medical devices is undeniably linked to simulation, and SIMULIA is leading the way in transforming how these life-saving technologies are developed.